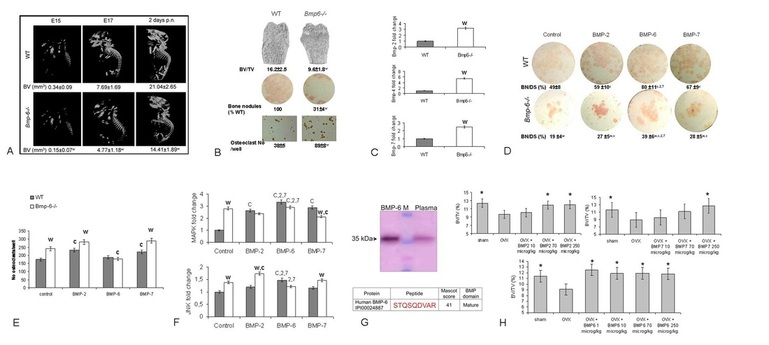
Results:
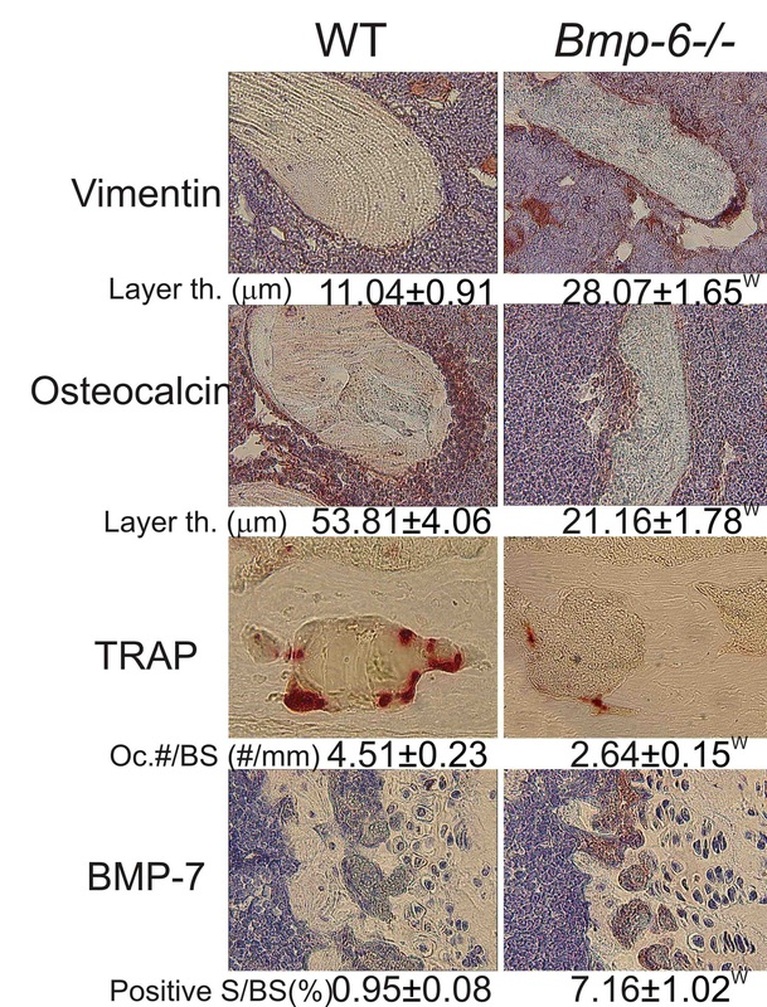
We showed that systemically administered BMP-6 restores bone in aged ovariectomized (OVX) rats and is not synergistic with 17β-estradiol (E2). In this project we explored the function of endogenous BMP-6 using Bmp-6 knock out (Bmp-6 -/-) mice. We have shown that, although bones of the wild type (WT) and Bmp-6 -/- littermates are of similar size and shape, the bone volume (BV) was reduced up to 122% and 61% at embryonic day 15 and 17, respectively, indicating a delay in ossification and mineralization of the entire skeleton (Figure 1). After birth, overall BV remained 38% lower in 2 days old Bmp-6 -/- mice followed by a difference in trabecular BV (tBV) with 13.2% in WT vs 10.3% in Bmp-6 -/- sham adult animals (Figure 1). The decrease in tBV in Bmp-6-/- mice was due to low bone turnover, with reduced numbers of osteoblasts and osteoclasts leading to decreased bone formation. Increased expression of Bmp-2, -4 and -7 (2.5-6 fold), in the femurs of Bmp-6 -/- mice and increased concentrations of BMP receptors in mesenchymal cells suggested that the effects of depletion of BMP-6 could not be overcome by BMP-2, -4 or -7 (Figure 1). Although Bmp-6 -/- bones had more vimentin-positive undifferentiated mesenchymal progenitors, in vitro analyses of the primary cultures showed that in the absence of BMP-6 that these cells remained undifferentiated, as confirmed by reduced osterix and Runx2 expression and by a reduced immunostaining for osteocalcin (Figure 2).
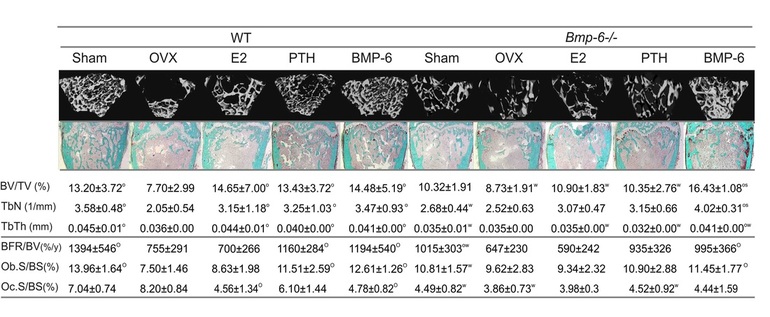
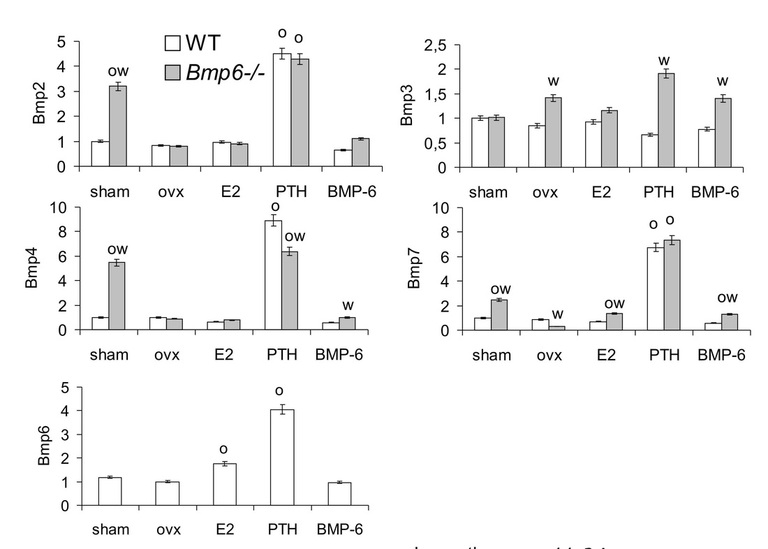
We also examined the role of endogenous BMP-6 in the pathogenesis and treatment of bone loss following ovariectomy in these mice. We ovariectomized (OVX) 4 months old Bmp-6 -/- and WT littermates to induce bone loss. After 3 weeks, the OVX mice were treated for 9 weeks with estradiol (E2) (50 μg/kg intraperitoneally 3xweek), as an antiresorptive agent, PTH (75 μg/kg subcutaneously 3xweek), as an anabolic drug, and BMP-2, -6 and -7 (10 μg/kg intravenously 3xweek each). Although OVX resulted in decreased expression of Bmp-2, -4 and -7 by 6-8 fold in femurs of Bmp-6-/- mice they had no additional tBV loss. Common therapies for bone loss, E2 and PTH, had no effect on the tBV of OVX Bmp-6 -/- femurs as shown by μCT and histology or on mesenchymal Bmp-6-/- stem cell differentiation (Figure 3). Although E2 therapy increased the mRNA expression of BMP-7 by 4.4 fold, and PTH dramatically increased the expression of BMP-2, -4 and -7 by 4-10 fold in femurs of Bmp-6 -/- OVX mice, these effects did not reverse the loss of tBV in Bmp-6 null mice. In the femurs of WT mice, E2 specifically up-regulated the expression of BMP-6 while PTH increased the expression of BMP-2, -4, -6 and -7 (Figure 4). Taken together, these results indicate that BMP-6 is required for the effects of both E2 and PTH on tBV and its deletion cannot be overcome by BMP-2, -4 and -7. Microarray analyses of femurs by Affymetrix gene chips also revealed increased levels of growth regulatory factors in Bmp-6-/- bones, such as FGF-1, IGF-1 and osteoprotegerin, indicating that these factors, like BMP-2,-4 and -7, could not overcome the loss of BMP-6. BMP-6 increased expression of extracellular matrix, cell cycle, growth factor/cytokine and transcription factor related genes, including procollagen type IX, IGF-1, EGF, interleukins 12 and 17.
To check whether the effect of BMP-6 on tBV is distinct from those of other BMPs, we analyzed the bones after the systemic administration of BMP-2, -6 and -7. Neither BMP-2 nor -7 in contrast to BMP-6 (at the same dose), was able to restore the tBV after OVX in WT and Bmp-6 -/- mice (Figure 1H). Exogenously administered BMP-6, in greater amount than BMP-2 and -7, transformed early bone marrow mesenchymal progenitors to bone forming cells (Figure 1D). BMP-6 is reported to act principally on osteoprogenitor cells at earlier stages, as compared to other BMPs.
After demonstrating a specific role for BMP-6 on systemic tBV, we have also tested its local function in the process of fracture healing. Fracture healing in Bmp-6 -/- mice was initiated, but the healing of femoral fractures was prolonged, with increased callus volume of 41.6% at 21 days following fracture as compared to WT mice, reaching values similar to those found in WT OVX animals (Figure 5). OVX decreases the protein concentrations of all BMPs leading to a delay in fracture healing, similar to that found in Bmp-6 -/- mice.
We showed that systemically administered BMP-6 restores bone in aged ovariectomized (OVX) rats and is not synergistic with 17β-estradiol (E2). In this project we explored the function of endogenous BMP-6 using Bmp-6 knock out (Bmp-6 -/-) mice. We have shown that, although bones of the wild type (WT) and Bmp-6 -/- littermates are of similar size and shape, the bone volume (BV) was reduced up to 122% and 61% at embryonic day 15 and 17, respectively, indicating a delay in ossification and mineralization of the entire skeleton (Figure 1). After birth, overall BV remained 38% lower in 2 days old Bmp-6 -/- mice followed by a difference in trabecular BV (tBV) with 13.2% in WT vs 10.3% in Bmp-6 -/- sham adult animals (Figure 1). The decrease in tBV in Bmp-6-/- mice was due to low bone turnover, with reduced numbers of osteoblasts and osteoclasts leading to decreased bone formation. Increased expression of Bmp-2, -4 and -7 (2.5-6 fold), in the femurs of Bmp-6 -/- mice and increased concentrations of BMP receptors in mesenchymal cells suggested that the effects of depletion of BMP-6 could not be overcome by BMP-2, -4 or -7 (Figure 1). Although Bmp-6 -/- bones had more vimentin-positive undifferentiated mesenchymal progenitors, in vitro analyses of the primary cultures showed that in the absence of BMP-6 that these cells remained undifferentiated, as confirmed by reduced osterix and Runx2 expression and by a reduced immunostaining for osteocalcin (Figure 2).
We also examined the role of endogenous BMP-6 in the pathogenesis and treatment of bone loss following ovariectomy in these mice. We ovariectomized (OVX) 4 months old Bmp-6 -/- and WT littermates to induce bone loss. After 3 weeks, the OVX mice were treated for 9 weeks with estradiol (E2) (50 μg/kg intraperitoneally 3xweek), as an antiresorptive agent, PTH (75 μg/kg subcutaneously 3xweek), as an anabolic drug, and BMP-2, -6 and -7 (10 μg/kg intravenously 3xweek each). Although OVX resulted in decreased expression of Bmp-2, -4 and -7 by 6-8 fold in femurs of Bmp-6-/- mice they had no additional tBV loss. Common therapies for bone loss, E2 and PTH, had no effect on the tBV of OVX Bmp-6 -/- femurs as shown by μCT and histology or on mesenchymal Bmp-6-/- stem cell differentiation (Figure 3). Although E2 therapy increased the mRNA expression of BMP-7 by 4.4 fold, and PTH dramatically increased the expression of BMP-2, -4 and -7 by 4-10 fold in femurs of Bmp-6 -/- OVX mice, these effects did not reverse the loss of tBV in Bmp-6 null mice. In the femurs of WT mice, E2 specifically up-regulated the expression of BMP-6 while PTH increased the expression of BMP-2, -4, -6 and -7 (Figure 4). Taken together, these results indicate that BMP-6 is required for the effects of both E2 and PTH on tBV and its deletion cannot be overcome by BMP-2, -4 and -7. Microarray analyses of femurs by Affymetrix gene chips also revealed increased levels of growth regulatory factors in Bmp-6-/- bones, such as FGF-1, IGF-1 and osteoprotegerin, indicating that these factors, like BMP-2,-4 and -7, could not overcome the loss of BMP-6. BMP-6 increased expression of extracellular matrix, cell cycle, growth factor/cytokine and transcription factor related genes, including procollagen type IX, IGF-1, EGF, interleukins 12 and 17.
To check whether the effect of BMP-6 on tBV is distinct from those of other BMPs, we analyzed the bones after the systemic administration of BMP-2, -6 and -7. Neither BMP-2 nor -7 in contrast to BMP-6 (at the same dose), was able to restore the tBV after OVX in WT and Bmp-6 -/- mice (Figure 1H). Exogenously administered BMP-6, in greater amount than BMP-2 and -7, transformed early bone marrow mesenchymal progenitors to bone forming cells (Figure 1D). BMP-6 is reported to act principally on osteoprogenitor cells at earlier stages, as compared to other BMPs.
After demonstrating a specific role for BMP-6 on systemic tBV, we have also tested its local function in the process of fracture healing. Fracture healing in Bmp-6 -/- mice was initiated, but the healing of femoral fractures was prolonged, with increased callus volume of 41.6% at 21 days following fracture as compared to WT mice, reaching values similar to those found in WT OVX animals (Figure 5). OVX decreases the protein concentrations of all BMPs leading to a delay in fracture healing, similar to that found in Bmp-6 -/- mice.
Figure 1. a/ µCT analyses of WT and Bmp-6 -/- embryos at day 15 (E15), 17 (E17) and at 2 days of postnatal (p.n.) life. b/ µCT of distal femurs, bone nodules (alkaline phosphatase staining) and osteoclasts (tartrate resistant acid phosphatase staining) from femoral bone marrow cells. c/ Real time PCR expression of Bmp-2,-4 and -7 in thefemur of WT and Bmp-6 -/- mice. d/ Mesenchymal progenitor cells stained for alkaline phosphatase positive bone nodules. e/ Number of osteoclasts from HSCs. f/ Real time PCR expression of signaling pathways in MSC. g/ Western blot and LC-MS detection of BMP-6 in pooled plasma of 30 healthy individuals. h/ BMP-2, -6 and -7 systemic therapy of OVX rats. BV/TV, bone volume/tissue volume; Co control, BN/DS bone nodule/disk surface, significant difference from control (c), BMP-2 (2), BMP-7 (7), OVX (*) and WT (w) (P<0.05, ANOVA Dunnett test).
Figure 2. Vimentin, osteocalcin, tartrate resistant acid phosphatase (TRAP) for osteoclasts, and BMP-7 immunohistochemistry of distal femurs (40x magnification). W, significantly different from WT (P<0.05, ANOVA Dunnett test).
Figure 3. µCT, static and dynamic histomorphometry (Goldner trichome staining, 10x magnification) of distal femurs. BV/TV bone volume/tissue volume, TbN trabecular number, TbTh trabecular thickness, BFR/BV bone formation rate/bone volume, Ob.S/BS osteoblast surface/bone surface, Oc.S/BS osteoclast surface/bone surface, th. thickness, Oc.#/BS osteoclast number/bone surface, S/BS surface/bone surface, O significantly different from OVX and W from WT (P<0.05, ANOVA Dunnett test).
Figure 4. Real time PCR expression of Bmps in thefemur. O significantly different from OVX and W from WT (P<0.05, ANOVA Dunnett test).
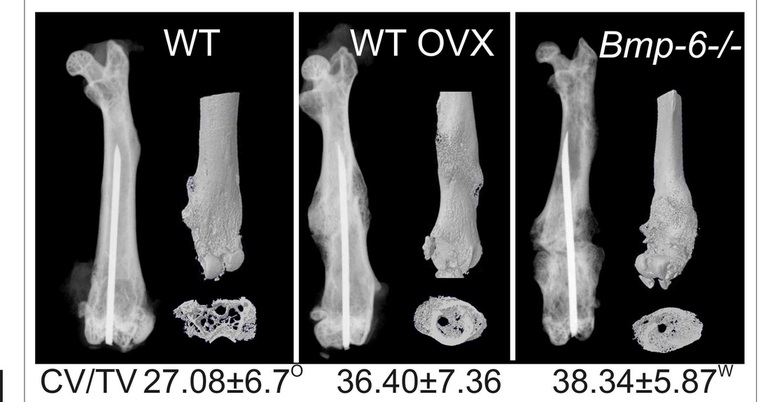
Figure 5. µCT analyses of WT and Bmp-6 -/- calluses at 21 days following the fracture. CV/TV callus volume/tissue volume. O significantly different from OVX and W from WT (P<0.05, ANOVA Dunnett test).